Molecule of the Month May 2019
Sucralose (and other sugar substitutes)
Molecule of the Month
Date: May 2019
Subject: Sucralose (and other sugar substitutes)
There is a wide variety of sugar substitutes on the market but they all have one thing in common: they provide a sweet taste similar to that of sugar but contain significantly less calories. Examples include Aspartame, Saccharin, Stevia, xylitol, sorbitol and the most widely used sweetener, sucralose.
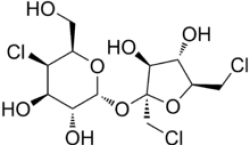
Sucralose is about 600 times sweeter than sugar and is produced through the chlorination of sucrose or raffinose. It is stable when heated, so in addition to being widely used in beverages, frozen desserts, chewing gums and other foods, it can also be used in baked goods and fried items. And due to the way it is metabolized in the body, it is quite safe to humans with only 15% actually being absorbed by the digestive system.
One of the main steps in the production of Sucralose is the substitution of three hydroxyl groups with chlorine atoms. This is accomplished through the phosgenation of sucrose, a reaction that is successfully performed in the Buss-Loop® Reactor. Indeed, Buss ChemTech participated in some of the process development work for Tate & Lyle, the discoverers of this molecule.
To learn more about producing Sucralose or other sugar substitutes, contact Thomas Blocher, Business Manager Reaction Technology at +41 61 825 6317 or by liam-e
External Input/sources for this Article: Wikipedia Article_Sucralose
